Interpreting UV-Vis Spectra
One of the most important factors affecting the wavelength of absorption by a molecule is the extent of conjugation. A conjugated diene is one that contains alternating double and single bonds. One characteristic is that they are more stable than their non-conjugated counterparts. As the extent of conjugation increases, the energy difference between HOMO and LUMO decreases. This means that with a greater extent of conjugation, less energy is needed (and the longer the wavelength of radiation) to excite an electron for the л → л* transition, so that extensively conjugated compounds can absorb in the visible region of the electromagnetic spectrum and they appear colored. Thus, by measuring the UV spectrum of an unknown, we can derive structural information about the nature of any conjugated л electron systems present in the molecule. As a first example, compare the MO diagrams for ethylene and 1,3-butadiene shown below.
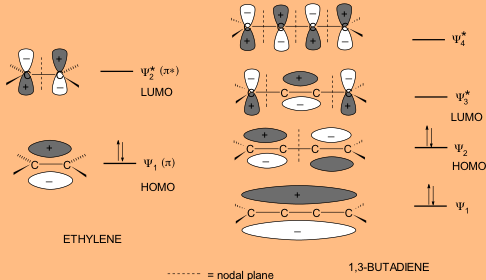
Figure 7: Energy difference between HOMO and LUMO in conjugated versus non-conjugated systems
Further evidence of this effect is shown below. From the polyene spectra displayed below in Figure 8, it is clear that each additional double bond in the conjugated pi-electron system shifts the absorption maximum about 30 nm in the same direction. Also, the molar absorptivity (ε) roughly doubles with each new conjugated double bond. Thus, extending conjugation generally results in bathochromic (to longer wavelength) and hyperchromic (to greater absorbance) shifts in absorption.
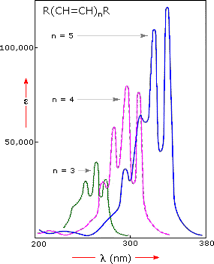
Figure 8: Effect of conjugation on λmax
The appearance of several absorption peaks (or shoulders) can be seen in Figure 8 above. This phenomenon is common for highly conjugated systems, though it is often solvent dependent. This fine structure reflects not only the different 3-dimensional conformations such systems may assume, but also electronic transitions between the different vibrational energy levels possible for each electronic state. Vibrational fine structure of this kind is most pronounced in vapor phase spectra, and is increasingly broadened and obscured in solution as the solvent is changed from hexane to methanol.
Many other types of conjugated pi-electron systems absorb light in the 200 to 800 nm region. These include unsaturated aldehydes and ketones and aromatic ring compounds. Below is a table of some characteristic UV absorptions for various chromophores, followed by several representative spectra.
Table 1
| Chromophore | Example | Excitation | λmax, nm | ε | Solvent |
| C=C | Ethene | π → π* | 171 | 15,000 | hexane |
| C≡C | 1-Hexyne | π → π* | 180 | 10,000 | hexane hexane |
| C=O | Ethanal | n → π* π → π* |
290 180 |
15 10,000 |
hexane |
| N=O | Nitromethane | n → π* π → π* |
275 200 |
17 5,000 |
ethanol ethanol |
| C-X X=Br, I | Methyl bromide Methyl iodide |
n → σ* n → σ* |
205 255 |
200 360 |
hexane hexane |
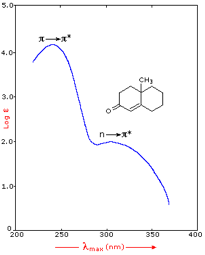
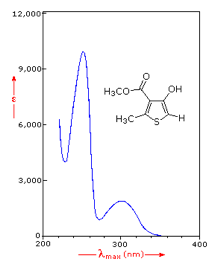
Figure 9: Effect of conjugation on λmax
A set of empirical rules has been developed for predicting the λmax of chromophores (Table 2). To calculate the λmax, the base value is added to the values listed for each particular functional group present in the molecule.
Woodward-Fieser Rules for Calculating the λmax of Conjugated Dienes and Polyenes
Table 2
| Core Chromophore | Substituent and Influence |
| R- (Alkyl Group) .... +5 nm RO- (Alkoxy Group) .. +6 X- (Cl- or Br-) ......... +10 RCO2⁻ (Acyl Group) .... 0 RS- (Sulfide Group) .. +30 R2N- (Amino Group) .. +60 Further π -Conjugation C=C (Double Bond) ... +30 C6H5 (Phenyl Group) ... +60 |
|
(i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (ii) Solvent effects are minor. * When a homoannular (same ring) cyclohexadiene chromophore is present, a base value of 260 nm should be chosen. This includes the ring substituents. Rings of other size have a lesser influence. |
|
λmax (calculated) = Base (215 or 260) + Substituent Contributions
Woodward-Fieser Rules for Calculating the π → π* λmax of Conjugated Carbonyl Compounds
| Core Chromophore | Substituent and Influence |
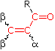 R = Alkyl 215 nm
R = Alkyl 215 nmR = H 210 nm R = OR' 195 nm |
α- Substituent R- (Alkyl Group) +10 nm Cl- (Chloro Group) +15 Br- (Chloro Group) +25 HO- (Hydroxyl Group) +35 RO- (Alkoxyl Group) +35 RCO2⁻ (Acyl Group) +6 β- Substituent R- (Alkyl Group) +12 nm Cl- (Chloro Group) +12 Br- (Chloro Group) +30 HO- (Hydroxyl Group) +30 RO- (Alkoxyl Group) +30 RCO2⁻ (Acyl Group) +6 RS- (Sulfide Group) +85 R2N- (Amino Group) +95 γ & δ- Substituents R- (Alkyl Group) +18 nm (both γ & δ) HO- (Hydroxyl Group) +50 nm (γ) RO- (Alkoxyl Group) +30 nm (γ) Further π -Conjugation C=C (Double Bond) ... +30 C6H5 (Phenyl Group) ... +60 |
Cyclopentenone 202 nm |
|
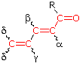 |
|
(i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (ii) Homoannular cyclohexadiene component adds +35 nm (ring atoms must be counted separately as substituents) (iii) Solvent Correction: water = –8; methanol/ethanol = 0; ether = +7; hexane/cyclohexane = +11 |
|
λmax (calculated) = Base + Substituent Contributions and Corrections