Solubility Theory
Solubility plays a critical role in organic chemistry. Its applications are extensive, ranging from purification (extraction) to identification of unknown compounds. It is the latter that will be discussed. Using solubility as a means of identification takes advantage of the properties associated with certain functional groups. While it does not determine the exact chemical structure, as spectroscopy techniques do, it provides a rough idea of key functional groups present and the degree of hydrocarbon character.
What is solubility?
Solubility can be defined as the maximum amount of solute that can dissolve in a fixed amount of solvent at a specific temperature. The solute is the dissolved substance while the dissolving substance is the solvent.
What is considered “dissolved”?
A solute is considered “dissolved” when a homogenous solution is formed, with no suspended particles. If the solute is liquid, there should be no distinguishable layers between solute and solvent. (Lab Tip: put a dark sheet of paper behind test-tubes when checking for solubility.)
What takes place at a molecular level?
The formation of a solution is a 3 step process that can be represented by an enthalpy diagram:
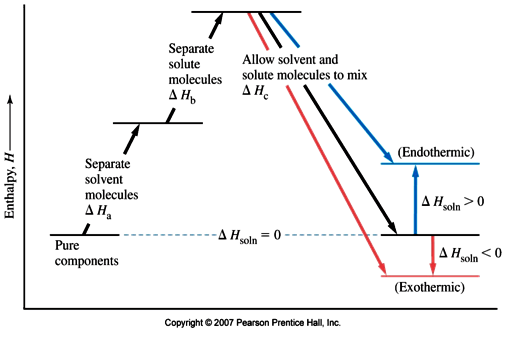
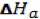 Breaking of solvent-solvent interactions
Breaking of solvent-solvent interactions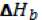 Breaking of solute-solute interactions
Breaking of solute-solute interactions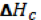 Formation of solute-solvent interactions
Formation of solute-solvent interactions
Steps 1 and 2 are endothermic processes because energy is required to break up molecular interactions (these interactions could be hydrogen bonds, dipole-dipole interactions, dispersion forces, etc). The enthalpy change, and thus, the outcome of the solution process depends on the attraction of solute to solvent.
If the solute-solvent interactions are either EQUALLY FAVOURABLE OR MORE FAVOURABLE than the
intramolecular interactions, the solute will be dissolved.
If the intermolecular interactions are LESS FAVOURABLE than the intramolecular interactions,
the solute WILL NOT dissolve.
Example: Acetone being dissolved in water
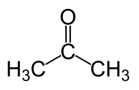 Acetone molecules have a polar carbonyl group that allows them to ACCEPT hydrogen bonds from OTHER compounds.
There are no polar C-H or O-H bonds on acetone; therefore, it cannot form hydrogen bonds
with other acetone molecules. The intramolecular forces consist of dispersion forces.
Acetone molecules have a polar carbonyl group that allows them to ACCEPT hydrogen bonds from OTHER compounds.
There are no polar C-H or O-H bonds on acetone; therefore, it cannot form hydrogen bonds
with other acetone molecules. The intramolecular forces consist of dispersion forces.
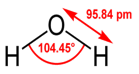 Water, on the other hand, has two polar O-H bonds. The slightly positive charge on each hydrogen can attract
slightly negative oxygen atoms on other water molecules, forming hydrogen bonds.
Water, on the other hand, has two polar O-H bonds. The slightly positive charge on each hydrogen can attract
slightly negative oxygen atoms on other water molecules, forming hydrogen bonds.
If acetone is added to water, acetone would completely dissolve.
WHY?—The carbonyl group on acetone would be able to form a hydrogen bonds (much stronger than dispersion forces).
There would be no change in the type or amount of hydrogen bonding that would take place from the perspective of water.
This results in a more favourable solute-solvent interaction, so acetone goes into solution.
For a solution to form: Intramolecular forces < Intermolecular forces
Factors affecting Solubility and Rate of Solvation:
- Polarity of solute — this is determined by the functional groups present on the compound
- Temperature — increasing temperature means higher solubility because it supplies the energy required to overcome the unfavourable interactions of solute and solvent
- Agitation — allows the solute and solvent to come in contact for intermolecular interactions to form
- Surface Area of solute — a higher surface area means more sites for interactions to occur
- Time!!! — make sure to give time (~5minutes) for the solution process to take place
- This is important when testing unknowns to prevent inaccurate results
Solubility Application - Solubility by Reaction
Solubility by reaction takes advantage of the presence of a functional group and the properties associated with it. Organic acids and bases may reaction to form water soluble salts, thus forcing the compound into solution. The tests outlined in the chart (in Lab manual) provide a means by which key functional groups and extent of hydrocarbon structure can be determined.
Performing solubility tests
Some things to keep in mind while performing solubility tests:
- Keep bottle lids closed — the solvent can absorb water in the air and make it more polar, giving misleading results
- Use clean and dry glassware — rinse the test tube beforehand with the solvent that will be used immediately after
- Proportion of solute to solvent
- Why? Often times, students will add too much solute to a small amount of solvent, forming a supersaturated solution — the compound is actually soluble, but because the capacity of the solvent is exceeded, no more solute can be dissolved. It appears as if the compound is insoluble.
- Practice the appropriate proportion of solute to solvent to prevent misleading results. Try testing the solubility of a known compound where the results can be predicted to get the proportions correct. Generally, a spatula tip (or 2 drops) of solute to 20 drops of solvent will suffice.
- Do not perform all tests — the purpose of these sequential tests is to draw conclusions at each step which will guide which tests to perform after.
Tests
- Room temperature water (polar solvent) — this test gives a quick idea of the polarity and the number of carbons in the compound.
- If soluble: the compound is polar and has low carbon number (less than 5 carbons). To test
the characteristics (acid, base, neutral) of any functional groups present, use litmus paper.
- Acidic — the compound is most likely a carboxylic acid. Carboxylic acids are acidic (pKa~5) because the conjugate base formed is resonance-stabilized through the carbonyl group.
- Basic — the compound is most likely an amine. Amines (RNH2, R2NH, R3N) are able to accept protons due to the lone pair of electrons on the nitrogen atom. The lower electronegativity also allows nitrogen to accommodate the positive charge following protonation.
- Neutral — the compound is most likely an alcohol. The compound does not lose a proton (=acidic) because the resulting alkoxide cannot stabilize the negative charge. It does not gain a proton readily because oxygen’s electronegativity destabilizes the positive charge that would result from protonation.
- If insoluble: heat the mixture and note if it dissolves. If so, test pH and use these conclusions to decide which additional tests are required to confirm this finding.
- If insoluble in hot and cold water: proceed to the right hand side of the chart.
- Conclusions that can be drawn — high carbon number (#C>4), but nothing can be concluded regarding functional groups. This is why additional tests are required.
- If soluble: the compound is polar and has low carbon number (less than 5 carbons). To test
the characteristics (acid, base, neutral) of any functional groups present, use litmus paper.
- Diethyl Ether (only if soluble in water) — the purpose of this test is to distinguish between inorganic salts and organic compounds.
- If soluble: the compound is ORGANIC because the hydrocarbon portion, however small, interacts with ether
- If insoluble: the compound is INORGANIC because it has no hydrocarbon portion to put it into the solution.
- 5% HCl — this test checks for the presence of basic functional groups using a strong acid.
- If soluble: most likely an amine with high carbon number.
- Why? An acid base reaction can take place, forming an organic salt, which is now soluble in water.
Therefore, the unknown compound can now go into the solution.
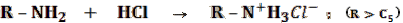
- Why? An acid base reaction can take place, forming an organic salt, which is now soluble in water.
Therefore, the unknown compound can now go into the solution.
- If insoluble: The compound is either acidic or neutral with high carbon number. More testing is required to determine the acidity or neutrality of any functional groups.
- If soluble: most likely an amine with high carbon number.
- 5% NaOH — this test checks for the presence of any acidic functional groups using a strong base.
- If soluble: the compound could be EITHER a high carbon number carboxylic acid or a phenol.
Subsequent tests are required to determine the degree of acidity.
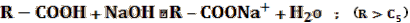
- If insoluble: the compound is neutral with high carbon number. Sulphuric acid test is required.
- If soluble: the compound could be EITHER a high carbon number carboxylic acid or a phenol.
Subsequent tests are required to determine the degree of acidity.
- 5%NaHCO3 — this test essentially checks for HOW acidic the compound is. Sodium bicarbonate is a weak base,
and the neutralization reaction will only go to completion if it reacts with a strong acid.
- If soluble: the compound is most likely a high carbon number carboxylic acid.
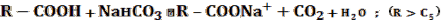
- If insoluble: the compound is most likely phenol
Why is a carboxylic acid more acidic than phenol?
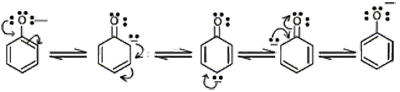 If phenol loses a proton, the resulting resonance structures on the phenoxide form negative charges on carbon atoms.
In the carboxylate ion, the resonance structures result with electron density being localized onto oxygen.
Oxygen is more electronegative than carbon, so it can accommodate the additional negative charge more effectively,
making it a weaker conjugate base, and thus stronger acid.
If phenol loses a proton, the resulting resonance structures on the phenoxide form negative charges on carbon atoms.
In the carboxylate ion, the resonance structures result with electron density being localized onto oxygen.
Oxygen is more electronegative than carbon, so it can accommodate the additional negative charge more effectively,
making it a weaker conjugate base, and thus stronger acid.
- If soluble: the compound is most likely a high carbon number carboxylic acid.
- H2SO4 — this test will differentiate between carbonyl compounds and purely hydrocarbon compounds.
- If soluble: the compound has a carbonyl group or some degree of unsaturation. It could be an aldehyde, ketone, ester, amide, or unsaturated compound.
- If insoluble: the compound has no polar functional groups, and thus does not react with the acid. Therefore, it is most likely a large hydrocarbon structure.
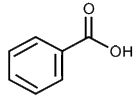
Example
Benzoic Acid—What tests would you perform, and what results would you anticipate?