Melting Point Theory
Melting Point Determination
The melting point of a substance is the temperature at which crystalline
substances change from a solid to a liquid state. During the melting process,
all of the energy added to a substance is consumed as heat of fusion, and the
temperature remains constant. A pure substance melts at a precisely defined
temperature, characteristic of every crystalline substance. With a pure
substance, a melting point is the quickest and most accessible method for an
organic chemist to confirm the identity of a compound. Additionally, it can
also be used as a way to assess the purity of a product by comparing measured
melting points to known literature values. At the melting point,
the solid and liquid phase exist in equilibrium. Thus the melting point depends
on pressure and usually reported at standard pressure.
Instructions:
At UTSC, the equipment
used to measure melting point is called the “Melting Point System MP50”. The
apparatus consists of four test tube slots in which substances can be tested as
well as a screen where individuals can adjust key criteria with respect to
their substance.

First of all, you would
take a sample of your solid out of your sample bag or from an unknown sample that
has been given to you by your TA, and using the capillary tube, obtain a small
quantity of the fine powder by gently rolling the tube through the substance.

If your product
particles are too large to fit into the capillary tube, place it in a mortar
and crush it into a fine powder.

Secondly, to lower the
substance to the bottom of the tube, either tap the closed end of the capillary
tube vigorously on the bench top, or drop the tube into the long clear plastic
tube and allow it to bounce on the bench top. Ensure that there is
approximately 3mm of sample within the tube.


Then, press the method
icon on the MP50 machine as has been instructed by your TA.
Subsequently, insert
the capillary tube into the sample holder of the machine. Press the start
button, and wait for the melting point to appear on the screen. You can view
your capillary and solid melting on the screen of the machine.

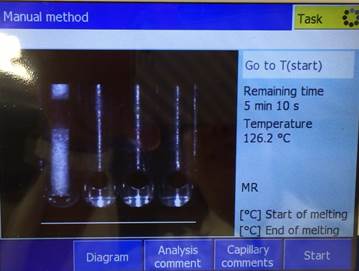
Record the melting
point of your substance within your notebook and clear the screen by pressing
the home button for the next student. After finding your melting point value,
proceed to compare it with documented known melting point values. This will assist
you in determining what compound you have. The machine will usually give you a
range for the melting point, with a starting value when the solid starts to
melt, and an end value for when all the solid has melted. Be sure to record
these numbers as a range for reporting your melting point.
Once you have your
melting point and determine what compound you have, you can continue to
complete your analysis of the lab. Based on the melting point obtained, you can
determine if any impurities exist in your sample. If your melting point is much
lower and a wider range than the literature value, impurities are present in
your sample. These can be due to experimental errors that occurred within your
experiment. Ensure to note these observations in the discussion of your lab
notebook.