Polarimetry
Background:
Polarimetry machines
are used in chemistry in a variety of ways. Their primary use is to measure the
angle of rotation of an optically active substance using polarized light. The
polarized light will either rotate clockwise or counter-clockwise and the
amount it rotates indicates the angle of rotation. Polarimetry is important in
chemistry due to the fact that it allows one to distinguish between optically
active stereoisomers using optical activity as a measuring point. There are
other key tests that are used in chemistry for identification of substances,
such as melting point. However, these tests would prove non conclusive for
identification of some stereoisomers, such as enantiomers, as they will have
identical physical properties, such as melting points and boiling points. The
step by step process of rotation of plane-polarized light in a polarimeter is
depicted by the schematic diagram below:
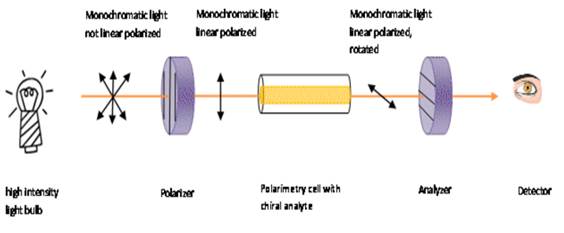
The specific rotation
value of a chiral substance is dependent on numerous variables. The main
variables are concentration of the substance and the length of the tube in the
polarimeter machine. This can be seen in the following equation:

Instructions:
First, prepare a
solution of your unknown substance with an appropriate solvent and note its
concentration. To prepare the solution using your unknown substance, weigh out
the desired mass of substance and using a funnel, transfer it to a volumetric
flask. Using an appropriate solvent in which your compound dissolves in, fill
the volumetric flask to its midpoint.

Next, rinse the
weighing tray and funnel with a glass pipette using the same solvent. Fill the volumetric flask up to the line with
a glass pipette. Stopper the flask. Dissolve the solid by shaking the
volumetric pipette up and down.

Next, ensure that the
polarimeter sample holder is clean.
Note that there are
three sections to the polarimeter sample holder: the silver cap, the black
washer and a clear lens. Be careful not to break the lens.

Following this, rinse
the sample holder with the solvent you have made your solution in, and then
empty into a waste beaker.
Next, use the solvent
you have made your solution in to calibrate the machine. To do this, fill the
sample holder to the top with the respective solvent. Ensure no bubbles are
present; if there are bubbles present, tilt the sample holder to ensure the
bubbles are in the curved part of the holder and not in the top or bottom of
the sample holder.

Open the hatch of the
polarimeter and place the sample holder into the tube opening and then close
the hatch again.
Now, look through the
eyepiece and ensure light is visible. To calibrate the machine, ensure that
there is a uniformly-colored circle visible through the eyepiece.

Note, that the inner
and outer zeros on the Vernier scale are aligned, which shows that the machine
has been calibrated.

Next, discard the solvent
from the sample holder in the waste beaker and fill the sample holder with your
prepared solution. Ensure minimal bubbles are present, and that any bubbles are
in the curved portion of sample holder.
Now, place the sample
holder back in the machine and begin to take the reading. You should observe a
half-dark circle on the left and a half-dark circle on the right when you
rotate the dial in opposite directions.
Following this, adjust
the dial so that one full uniform light circle is seen. Now, you can read the
observed degree of rotation using the numbers on the outer dials. The outer
scale is the whole number for observed rotation reading and the inner scale is
the decimal point for the observed rotation reading.
To make the reading,
note where the zero on the inner scale meets up with the numbers on the outer
scale. The whole number in this example
is 1. To note the decimal position, see where a line in the inner scale matches
up with a line on the outer scale. The
decimal in this example is 1. So in this example, the reading is 1.1.


To determine if the
rotation is positive or negative, look at the position of the zero on the inner
scale. If zero on the inner scale sits below zero on the outer scale, this
means you have a sugar that has a negative degree of rotation. To complete this
reading, take the number you obtain and subtract it from 180 degrees or just
note the negative value. To find the decimal, again find a line from the inner
scale that matches that of the outer scale.
When you find your
observed optical rotation value, you are able to solve for the specific
rotation using the equation given above. Divide this observed optical rotation
value by the concentration of your solution and the length of the tube.
This final optical rotation reading can be used to compare to known literature values to determine what the unknown compound at hand is. As can be seen from this tutorial, polarimetry is an efficient and useful technique used in the field of chemistry for the identification of chiral molecules with optical activity, such as enantiomers and diastereomers. Polarimetry can also be used for determining the enantiomeric excess of a mixture of enantiomers. At the end of your experiment, ensure all utensils used are properly sterilized and returned to the designated location. Discrepancies in your findings with literature values can be a result of experimental error.