The Chemistry of Colour
Early humans valued colored pigments, and used them for decorative purposes. Many of these were inorganic minerals, but several important organic dyes were also known. These included the crimson pigment, kermesic acid; the blue dye, indigo; and the yellow saffron pigment, crocetin. A rare dibromo-indigo derivative, punicin, was used to color the robes of the royal and wealthy. The deep orange hydrocarbon carotene is widely distributed in plants, but is not sufficiently stable to be used as permanent pigment, other than for food coloring. A common feature of all these colored compounds, displayed below, is a system of extensively conjugated pi-electrons.
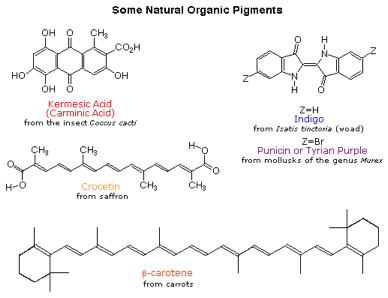
Figure 10: Examples of colour pigments
Some organic compounds are coloured and some are not. β-carotene, for example, is orange while cholesterol is colourless. The reason for these differences involves both the chemical structures and the way we perceive light.
Recall that the visible region of the electromagnetic spectrum is adjacent to the UV region, extending from approximately 400 to 800 nm. Coloured compounds have such extended systems of conjugation that their “UV” absorptions extend into the visible region. For example, when white light strikes β-carotene, the wavelengths from 400-500 nm (blue) are absorbed by its extensive pi-system while the other wavelengths are transmitted. These transmitted wavelengths reach our eyes causing us to perceive a yellow-orange colour of β-carotene.
Table 3 – The relationship between light absorption and colour.
Conjugation is also crucial for the light-sensitive molecules on which our visual system is based. The key substance for vision is dietary β-carotene, which is converted to vitamin A by enzymes in the liver. Vitamin A is then oxidized to an aldehyde called 11-trans-retinal, and lastly isomerised by a change in geometry of the carbon 11 and carbon 12 double bond to produce 11-cis-retinal.
In the rod cells of the eye, 11-cis-retinal is converted into rhodopsin, a light-sensitive substance formed from the protein opsin and 11-cis-retinal. When light strikes the rod cells, isomerisation of the C11-C12 double bond occurs and trans-rhodopsin is produced. In the absence of light, this cis-trans isomerisation takes approximately 1100 years, but in the presence of light, it occurs within 200 femtoseconds (2 x 10-13 seconds). The visual cycle is a circular enzymatic pathway, which is the front-end of phototransduction. It regenerates 11-cis-retinal. The full cycle is shown in the diagram below.
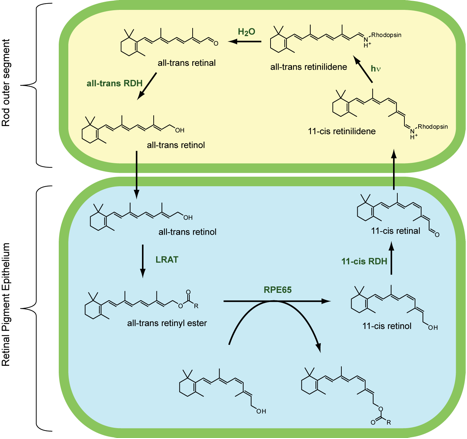 Vision Cycle
Vision Cycle