Rotary Evaporation Theory
What is rotary evaporation?
Rotary evaporation is a technique which employs a rotary evaporator (also called a “rotavap”) in order to remove excess solvents from samples by applying heat to a rotating vessel at a reduced pressure. An important concept that this technique applies is that liquids boil when the vapor pressure is equal to the external pressure or atmospheric pressure. The machine utilizes a lower pressure than atmospheric pressure which allows solvents to boil at lower temperatures. Furthermore, the rotation increases the surface area and therefore evaporation proceeds more rapidly.
Procedure and Setup
- Ensure that both the aspirator pump and the recirculating water bath (5 gallon bucket) are filled with ice.
- Check that the power strip is turned on and plugged in.
- Verify that the bump trap is clean and dry.
- Add your sample to a round-bottomed flask. (Note: The RB flask should not be more than half full with liquid.)

Correct (left) Incorrect (right) - Attach the round-bottom flask to the ground glass joint of the bump trap at the end of the distillation tube. (Note: Some assemblies will have an adapter between the bump trap and round-bottom flask.)
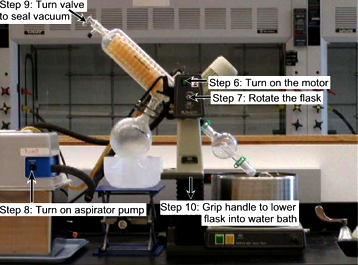
- Ensure that all glassware is held securely in place with a plastic Keck clip and/or ring cap.

- Turn on the rotary evaporator motor (green switch).
- Adjust the dial to rotate the flask at medium speed.
- Turn the aspirator pump.
- Seal the vacuum by closing the valve at the top of the diagonal rotary evaporator condenser.
(i.e. turn the knob until the arrow on it points straight down towards the attached tubing)

- If necessary, carefully lower the round-bottom flask into the water heating bath.
(Note: The hot water should cover the liquid level in the flask. If the RB flask is more than half-way full,
the water should touch the bottom of the RB flask and as the liquid evaporates, the RB flask can be further immersed in the water.)

- Stop the rotovap when there is no more liquid dripping from the condenser coils for 30 seconds.
(Note: For small volumes, dripping may not occur, wait 1-2 minutes and observe if there is any change.)

Shutdown (Essentially the reverse of setup)
- Lift the flask out of the water bath.
- Break the vacuum by opening the top valve at the top of the rotary evaporator condenser.

- Turn off the aspirator pump.
- Turn the flask rotation dial down to zero.
- Carefully remove the round-bottom flask.
Tips and general considerations
- Remember that a rotary evaporator evaporates solvent, which is very different from drying a solvent.
- Be sure to think about and understand the factors that contribute to the rapid evaporation of solvent in a rotary evaporator.
- The round-bottom flask should be no more than half full (half empty?). Large amounts of solvent can be removed either by transferring the solution to a larger flask or evaporating sequentially in smaller portions.
- It may be advantageous to know the mass of the clean, empty round-bottom flask prior to rotary evaporation.
- There is no need to turn off the rotary evaporator unless it is the end of the lab period.
Why is rotary evaporation important?
Rotary evaporation is useful for evaporating solvents that have high boiling points. This is because evaporating these solvents at atmospheric pressure requires high temperatures which may cause side reactions such as oxidation or decomposition of the compound to occur. Therefore, by lowering the pressure and boiling at a lower temperature, solvents with high boiling points are removed efficiently without the occurrence of unwanted side reactions.