Sublimation Theory
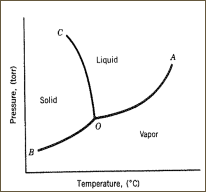
Sublimation is a phase transition process from a solid to a gas without ever entering an intermediate liquid phase. The ability of any types of solids to sublime depends on the compound’s triple point based on its phase diagram – typically the lower the pressure, the lower the sublimation temperature. (Note that the pressure and temperature of the desired substance must be below its triple point in order to sublime – Figure 1). Solid compounds that can sublime are very rare, for example, solid carbon dioxide (a.k.a. dry ice) can sublime at 1 atm pressure at 78.5 °C. For sublimation to occur, a solid must exhibit a higher than usual vapor pressure, i.e. it must have weak intermolecular attractions. This is normally true for solids with molecules in the shape of a sphere or a cylinder. The theory for process of sublimation lies in basic chemical properties. For example, higher temperatures result in a vapor pressure increase, i.e. the rate of evaporation is increased with heat. Moreover, a higher rate can be achieved if an evacuated system is used.
Sublimation is adopted by chemists as a purification technique. There are many advantages for performing sublimation over other purification methods. This process is principally used for micro scale purifications of solids because the loss of product is typically very minimal. Furthermore, this technique is appropriate for any heat sensitive compound (but under high vacuum, sublimation can be affected under low temperatures). Thirdly, unlike recrystallization, solvents are not involved at all in the process, and most traces of any solvent are effectively eliminated. However sublimation is only favored over crystallization, when the substance weighs less than 100mg, and has the correct properties. Based on the theory of this phase change, the process is highly dependent on the different vapor pressures of not only the desired compound, but also on the vapor pressures of the impurities that are present in the crude.
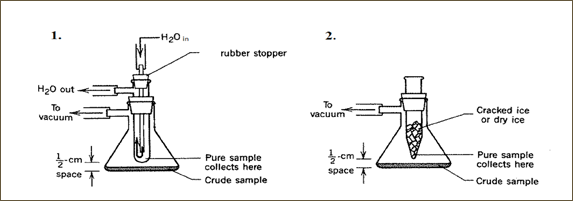
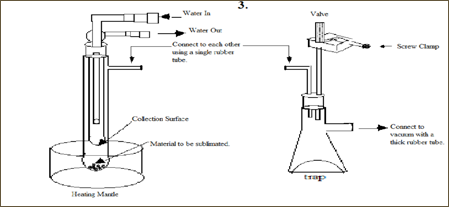
There are many ways to conduct sublimation. It can range from a very simple procedure to a more complicated one. A simple one would include only the use of two Petri dishes, a heat source and a beaker. This procedure entails having one of the Petri dishes placed on the bottom of the beaker with the crude material and the second dish situated on top of the beaker. Once the crude is heated, the sublimate or the purified material will appear on the top dish. Another simple method requires the use of any of the sublimation apparatus shown below. This method is carried out but heating the crushed crude solid in a tube or a Buchner flask (both on top of a heating mantle), while simultaneously evacuating the system. The purified product will sublime on the cooled condenser – the second inner tube with a water aspirator or on the tube containing cracked or dry ice) – after sufficient heating is applied under reduced pressure. Before starting sublimation, it is important to ensure that the apparatus is set up properly – this technique is not an easy one and when done improperly, will typically result in major loss of product.
Caffeine (C8H10N4O2) belongs to a group of naturally occurring compounds, alkaloids. It is used in the medical field as a diuretic and a central nervous system stimulator. Other popular examples of alkaloids include nicotine and morphine. Caffeine is a compound that can either be naturally extracted or synthetically made. It is extracted from substances such as tea (2-5%), coffee (1-3%), cocoa, cola syrup, and tea dust.
References
1. Fessenden R. J.; Fessenden J. S. Techniques and Experiments for Organic Chemistry, pp.408-409
2. Mayo, D. W.; Pike, R. M.; Forbes, D. C. Microscale Organic Laboratory: With Multistep and Multiscale Syntheses, 5th ed.; pp. 111 – 113.
3. Williamson, K. L. Macroscale and Microscale Organic Experiments, 2nd ed.; pp. 121-122.