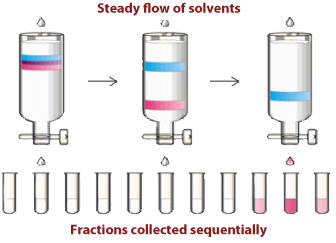
Column Chromatography Theory
Introduction
Chromatography (from Greek χρώμα:chroma, color and γραφειν:graphein to write) is the collective term for a set of laboratory
techniques for the separation of mixtures. It involves passing a mixture dissolved in a "mobile phase" through a stationary
phase, which separates the analyte to be measured from other molecules in the mixture based on differential partitioning
between the mobile and stationary phases. Subtle differences in a compound's partition coefficient result in differential
retention on the stationary phase and thus changing the separation.
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of
a mixture for further use (and is thus a form of purification). Analytical chromatography is done normally with smaller
amounts of material and is for measuring the relative proportions of analytes in a mixture. The two are not mutually exclusive.
What is column chromatography?
Column Chromatography is a preparative technique used to purify compounds depending on their polarity or hydrophobicity.
In column chromatography, a mixture of molecules is separated based on their differentials partitioning between a mobile
phase and a stationary phase. The different size column could be used for this technique.
Setting up a microcolumn (used for samples less than 200mg).
A pasteur pipette is used as the column and usually 1g of adsorbent (ie silica/alumina) is used to separate the mixture.
- Obtain a Pasteur pipette and ~1g of silica or alumina oxide.
- Setup the Pasteur pipette in such a way that it is straight and secure it with a clamp. A small amount of glass wool should be put into the base of the pipette using the other Pasteur pipette or wire. This is to prevent any of the fine particles from going through. Silica was then added to the pipette via the short stem funnel. The top of the silica must be flat/level. If the silica is not flat, then gently tap the pipette on a bench until it levels (this technique calls dry loading).
- You can use column with a small clamp to make a stopcock to control the flow rate (not necessary in our case).
- Small amount of sand could be added at the top of the column (about 2 mm high) to help the sample loading (it will help sample to go smoothly through the column). In this case the sand layer at the top must be flat as well.
- Wet the column using appropriate solvent/solvents (hexanes in the video case). It helps to compact the column (wet loading). The column cannot be dry at this point (you should continually add solvent to the column and keep its surface wet until you finish experiment).
- Collect the fractions using labelled test tubes or vials.
- Put the column in a container labelled PASTEROUS PIPETTES WASTE.

Column Chromatography Procedures
Loading the microcolumn
- Obtain the mixture to be separated and then make sure that it is less than 200mg. If it is over, use only 200.
- Dissolve the mixture with an appropriate solvent. Use as little solvent as possible in order to aid loading and to have a thinner band while running the column (you want this for good separation).
- When the solvent level is equal to that of the sand, then the solvent should be added to the column using another glass pipette. Ensure that the sample is added evenly onto the column so that there is a thin band for separation.
Running the microcolumn
- Once the sample has gone completely through the sand and is nearly touching the adsorbent, add the elution solvent to the column gently as to minimize the disturbance to the solid phase & sand.
- Keep adding elution solvent to the column such that it remains wet throughout the process of collection/elution.
Collecting fractions for the microcolumn
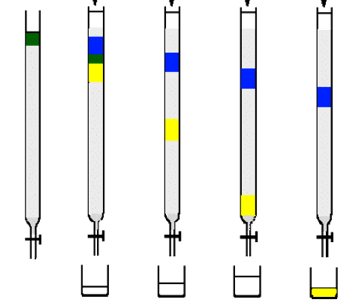
- As soon as the elution solvent is added, use test tubes to collect fractions and TLC to analyze the collected fractions. (To make sure you have the product you want) Also change the test tubes more frequently when closely associated bands are coming out of the column to get proper separation.
- Also label your fractions that have been collected.
What do we use to set up the column? (standard protocol)
Column chromatography consists of two distinct phases: the solid phase and the mobile phase. Usually Silica or Alumina are used as the solid phase in order to setup the column . In this experiment, we used Silica as the solid medium. In order to set up the column, a slurry consisting of the adsorbent along with an appropriate solvent, usually a non-polar solvent, is mixed together. The slurry needs to be stirred well in order to remove all the air bubbles otherwise the sample will not run evenly in the column. The prepared slurry is then added to the column. Sand is also needed at the top to aid in loading the sample.
How much Silica should be used?
Generally we use the following equation: Will have this shortly due to technical errors Since we are in the milligram scale, we will not need much silica, so we decided to separate with a micro column.
Preparing the column:
The microcolumn can be prepared using a Pasteur pipette. Glass wool is inserted at the bottom of the Pasteur pipette to prevent the silica from escaping the column. Make sure the bottom of the column is flat to ensure an even separation. Silica is then added to the column. Ensure that the top of the silica column is also level other wise it may result in an uneven separation. Use a metal spatula to tap the column until the silica level is flat. On the top, sea sand is added to ensure the extract runs at the same time. The column should be washed with the non-polar solvent in order to compact the silica. Once the column has been wetted with the solvent, it must remain wet. Keep adding solvent to the column and never let it dry. If the column dries up, the separation will be uneven leading to inaccurate results.
Choosing the right Solvent to run the column
Usually a nonpolar solvent is used to load the column when using silica as solid medium . Before using your eluent solvent, it should have been tested using the TLC to achieve an Rf value of around 0.3.
Loading the column
 When the solvent level has almost reached to the same height as the sand, then you may add the crude mixture
evenly at the top of the column. Make the band as thin as possible to ensure a good separation. Avoid
touching the solid silica medium when adding the crude mixture. Once the extracts reaches the silica,
you may add more solvent to elute.
When the solvent level has almost reached to the same height as the sand, then you may add the crude mixture
evenly at the top of the column. Make the band as thin as possible to ensure a good separation. Avoid
touching the solid silica medium when adding the crude mixture. Once the extracts reaches the silica,
you may add more solvent to elute.
Collecting fractions:
Make sure that there are enough test tubes to collect your compounds and enough solvent to run the column. Make sure to collect fractions as soon as the sample has been loaded because some compound may move at the same speed as the solvent. It is extremely important to take TLCs of the fractions to make sure that the right compounds are being isolated. Also ensure that your column never dries out during the collection process. When using a gradient column, it is important to start with the most non-polar solvent first and then to increase the polarity accordingly.