Infrared (IR) spectroscopy
Infrared (IR) spectroscopy is one of the most common spectroscopic techniques used by organic and inorganic chemists. IR spectroscopy comprises the region of the electromagnetic spectrum that is light with a longer wavelength and lower frequency than visible light. An IR spectrum is a plot of wave-number or wavelength (X-axis) vs. percent transmittance or absorbance (Y-axis). The spectrum has two regions. The fingerprint region is unique for a molecule and the functional group region is similar for molecules with the same functional groups. IR radiation is absorbed by organic molecules and converted into energy of molecular vibration, either stretching (produced by a change of bond length) or bending (resulted in change in bond angle). More energy is required to stretch a bond than to bend it.

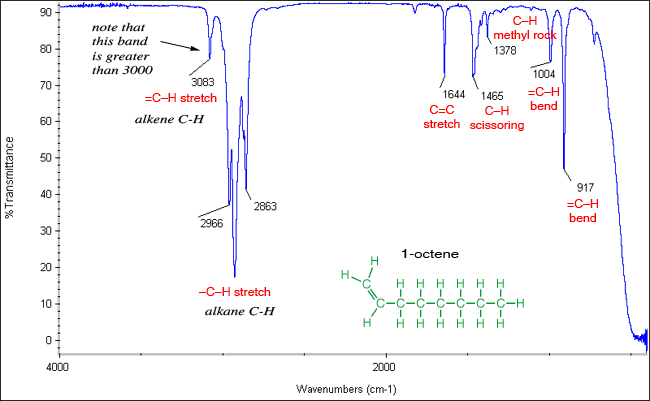
Absorption bands for stretching vibrations are found in the functional group region (4000 -14000 cm-1) whereas, absorption bands for bending vibrations are typically found in the fingerprint region (1400 -600 cm-1). A Carbon-Carbon triple bond is stronger than a double bond, so a triple bond stretches at a higher frequency (~2100 cm-1) than does a double bond (~ 1650 cm-1), as observed in the following diagram. The amount of energy required to stretch a bond depends on the strength of the bond and the masses of the bonded atoms. The stronger the bond, the greater the energy required to stretch it (analog to tighter spring). Lighter atoms show absorption bands at larger wavenumber such as C-H bond is found between 3300-2700 cm-1, C-O (1100 cm-1) and C-Cl (700 cm-1). Thus, we see inverse relationship between mass of the atom and the frequency of the vibration.
The trend in increasing bond strength, shorter bond, and thereby increasing wavenumber is shown in diagram 1 below. Therefore, the position, shape, intensity and absence of the absorption band, can all help in the compound identification process. It is important to note that IR radiation is not sufficient to excite electrons like the case with visible, ultraviolet, or higher energy forms of light. There are different types of bonds and thus different functional groups that absorb IR radiation of different wavelengths. Therefore, IR is used to gather information about compound's structure, assess its purity, and compound identification.
-
Spectra can be obtained by many methods. Solids can be measured as:
- KBr discs in which potassium bromide is powdered and combined with a small amount of sample and then pressed into a disc
- Mull, where the sample is powdered with a few drops of mineral oil and then spread between 2 KBr or NaCl plates
- Neat- just placing the sample on the diamond head of a reflectance infrared instrument
- Solutions- generally 5% made up in a deuterated solvent and placed into a NaCl cell
- Gases -in a larger glass cell with salt plates at either end.
NaCl and KBr are used because they are ionic crystals and due to their structure they will not absorb in the region you are looking at for organics. NaCl starts to absorb at about 700 cm-1 and KBr at about 400 cm-1 The advantages of infrared spectroscopy are that it is fast and relatively cheap and usually easily accessible in an undergraduate lab.
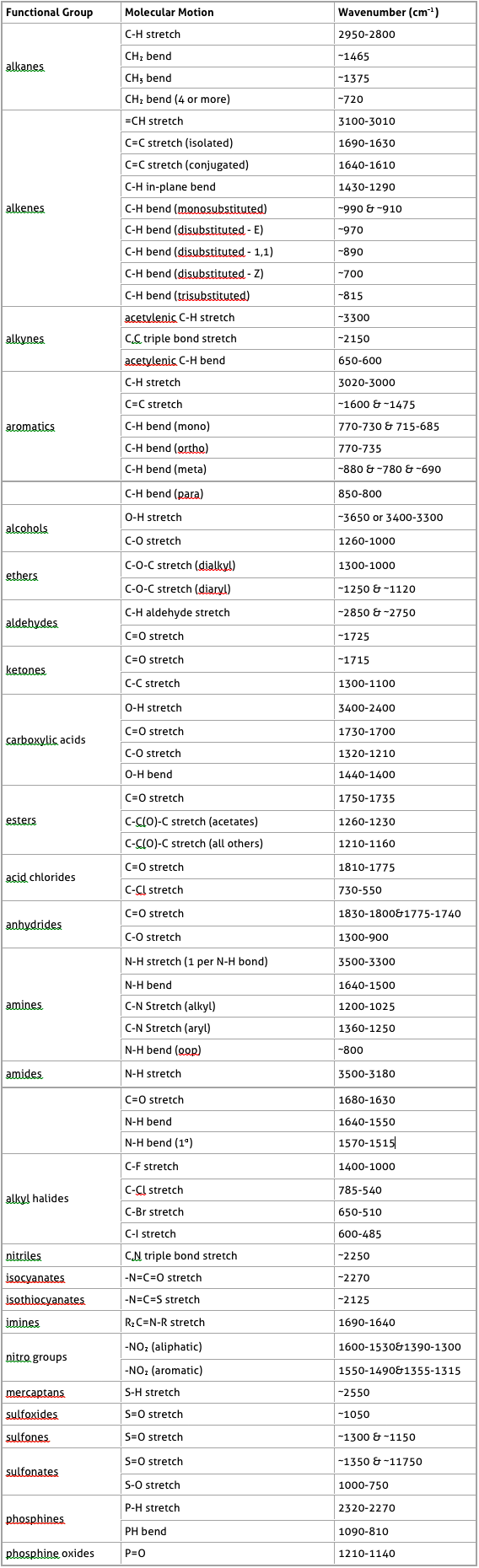
The following table was taken from http://www.chemistry.ccsu.edu/glagovich/teaching/316/ir/table.html
IR Absorptions for Representative Functional Groups